Most enterprises underestimate the role laboratory standards play in operational integrity. Testing results, calibration data, and inspection records form the evidentiary backbone of quality control, regulatory submissions, and supply-chain validation—yet these outputs are only as reliable as the laboratories producing them. In many cases, suppliers and regulatory authorities will not accept test or calibration results from a lab that is not accredited.
A common point of confusion: many enterprises mistakenly reference "ISO 17205" when searching for laboratory accreditation requirements. The correct standard is ISO/IEC 17025—the globally recognized international standard for testing and calibration laboratories. This distinction matters because accurate terminology signals operational sophistication when engaging vendors, auditors, and regulatory bodies.
This article defines ISO/IEC 17025, examines its core structural and technical requirements, identifies enterprise use cases where laboratory competence directly affects business outcomes, and clarifies why accreditation serves as a strategic risk-mitigation tool rather than a ceremonial credential.
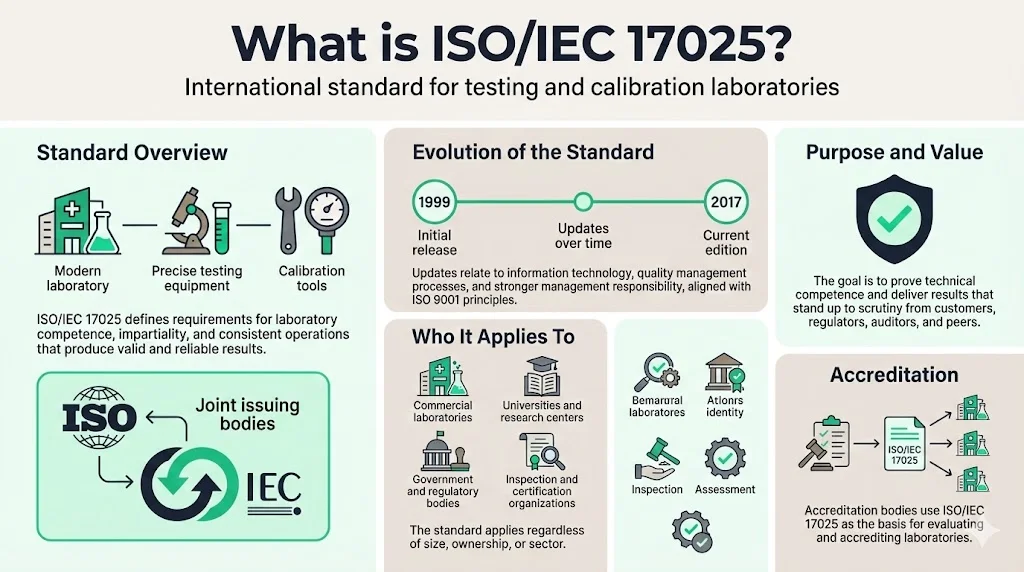
What is ISO/IEC 17025?
ISO/IEC 17025 is the international standard for testing and calibration laboratories. Issued jointly by the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC), the standard specifies requirements for demonstrating laboratory competence, impartiality, and the consistent operation necessary to produce valid, reliable results.
ISO/IEC initially issued the current version—ISO/IEC 17025 in 1999—has been updated twice. The latest revision includes updates on information technology, quality management system processes, and a stronger emphasis on the responsibilities of senior management. This 2017 edition represents the third iteration, addressing technological advances, evolving market conditions, and alignment with ISO 9001 quality management principles.
Ultimately, ISO 17025 is for any organization that performs testing, sampling or calibration and wants to be sure of the reliability of their results. The standard applies universally—regardless of laboratory size, ownership structure, or sector. The standard is also useful to universities, research centres, governments, regulators, inspection bodies, product certification organizations and other conformity assessment bodies with the need to do testing, sampling or calibration.
The fundamental purpose: enable laboratories to demonstrate technical competence and deliver results that withstand scrutiny from customers, regulators, auditors, and peer-review bodies. Accreditation bodies use ISO/IEC 17025 as the criteria for assessing and accrediting laboratories.
Core Structure & Requirements of ISO/IEC 17025
ISO/IEC 17025:2017 reorganized the standard's architecture from the 2005 version's bifurcated management-technical structure into an integrated framework addressing general, structural, resource, process, and management system requirements. Understanding this structure clarifies what laboratories must implement—and what enterprises should verify when evaluating laboratory vendors.
1) General & Structural Requirements
Laboratories must establish organizational accountability, document control mechanisms, and management responsibility frameworks. These foundational elements ensure quality assurance practices are institutionalized rather than ad hoc. For enterprises procuring inspection services, this translates to systematic documentation that supports audit trails and conformance verification.
2) Resource Requirements
This section also covers personnel, equipment, environmental and facility conditions and metrological traceability. Laboratories must demonstrate that staff possess appropriate competence, testing environments maintain specified conditions, and equipment undergoes regular calibration. A key distinction however is the greater emphasis on some requirements such as sampling (7.3), metrological traceability, which has a new sub clause of its own (6.5) under Resource Requirements—reflecting industry recognition that traceability to national and international measurement standards forms the basis for defensible results.
3) Process Requirements (Testing / Calibration / Sampling)
This clause constitutes the technical core of the standard. This section also focuses on the selection and verification of various methods, method validation, sampling and the handling of calibration or test items. Laboratories must define and validate testing protocols, establish calibration methods with documented procedures, and implement sampling techniques that preserve result integrity. For enterprises, this ensures that measurement accuracy claims rest on documented, validated methods rather than institutional custom.
4) Technical Requirements: Equipment & Traceability
Laboratories still have to carry out equipment calibration and method validation, participate in interlaboratory comparisons via proficiency testing and estimate uncertainty of measurement. Equipment used for inspection and calibration must itself be calibrated, maintained, and traceable to recognized measurement standards. This requirement addresses a fundamental vulnerability: instruments that drift out of specification produce systematically flawed data, creating cascading quality control failures that only surface during regulatory audits or product failures.
5) Quality Assurance of Results
Internal quality control mechanisms, proficiency testing participation, and measurement uncertainty estimation constitute ongoing validation that results remain reliable over time. This requirement prevents laboratories from achieving initial accreditation then allowing processes to degrade—a pattern that undermines the value of certification for enterprise clients who assume consistent performance.
6) Management System Requirements
Finally, ISO/IEC 17025 covers management system requirements, such as system options. Document control, internal audits, management review cycles, and corrective/preventive action protocols ensure laboratories maintain audit readiness and continuously improve operations. These elements provide the infrastructure for conformance verification and support regulatory acceptance requirements.
Who Needs ISO 17025—and Why Enterprises Should Care
Enterprises interact with ISO/IEC 17025 accreditation in multiple contexts, often without recognizing how laboratory competence directly affects operational risk, regulatory compliance, and market access.
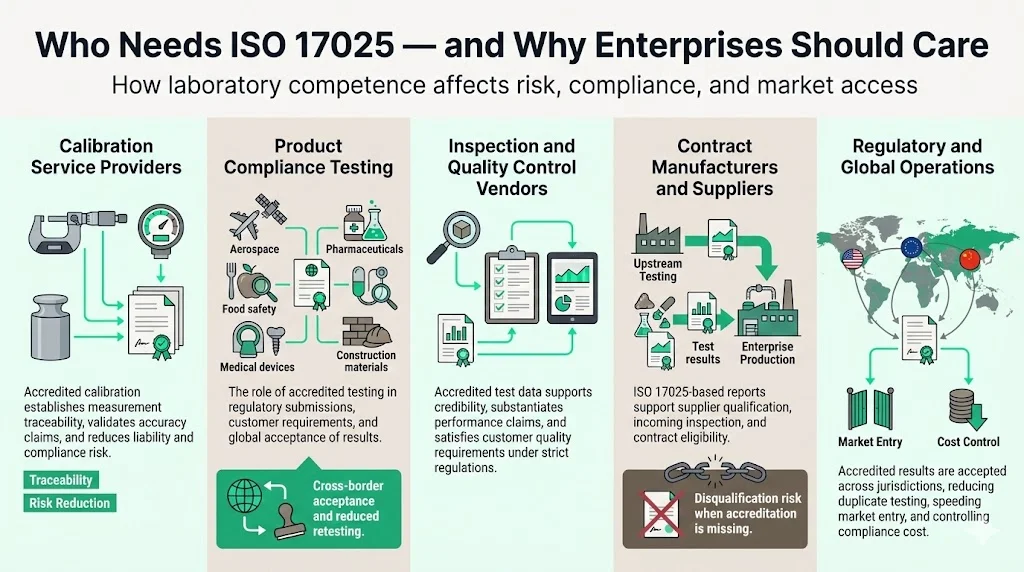
1) Calibration Service Providers
Organizations that supply calibrated measurement devices—from precision manufacturing equipment to environmental monitoring instruments—depend on ISO/IEC 17025-accredited calibration laboratories to establish traceability and validate accuracy claims. Without accredited calibration, manufacturers cannot demonstrate that inspection equipment meets specified tolerances, creating liability exposure and regulatory non-compliance.
2) Testing Laboratories for Product Compliance
Aerospace, pharmaceutical, chemical, food safety, medical device, and construction materials sectors require test results from accredited laboratories for regulatory submissions, customer specifications, and quality assurance documentation. By meeting a globally recognized standard, there will be wider acceptance of results across borders, meaning test reports and certificates can be accepted in one country from another without any need for further tests. This eliminates redundant testing costs and accelerates market entry timelines.
3) Inspection and Quality Control Vendors
Companies selling inspection services or inspection equipment face competitive pressure to substantiate performance claims. Providing test data and calibration certificates from ISO/IEC 17025-accredited laboratories signals technical credibility and supports customers' own quality assurance requirements. This becomes particularly critical when clients operate under stringent regulatory frameworks requiring documented measurement accuracy and traceability.
4) Contract Manufacturers and Suppliers
Enterprises outsourcing production increasingly require suppliers to provide ISO/IEC 17025-based test reports as part of incoming material inspection and supplier qualification processes. This requirement shifts quality control burden upstream while providing documentary evidence for conformance verification. Suppliers lacking access to accredited laboratories face disqualification from high-value contracts.
5) Regulatory Compliance and Cross-Border Operations
The acceptance of results between countries is facilitated if laboratories conform to this document. For enterprises managing international supply chains or exporting regulated products, ISO/IEC 17025 accreditation provides regulatory acceptance across jurisdictions. This reduces the need for parallel testing in each market, compressing time-to-market and controlling compliance costs.
Importance of Compliance & What Accreditation Means for Clients
ISO/IEC 17025 accreditation differs fundamentally from ISO 9001 certification. Laboratories are therefore "accredited" under ISO/IEC 17025, rather than "certified" or "registered" by a third party service as is the case with ISO 9000 quality standard. In short, accreditation differs from certification by adding the concept of a third party (Accreditation Body (AB)) attesting to technical competence within a laboratory—not merely adherence to documented procedures.
This distinction carries operational significance. In most countries, ISO/IEC 17025 is the standard for which most labs must hold accreditation in order to be deemed technically competent. Accreditation bodies conduct rigorous technical assessments evaluating both management systems and demonstrable measurement competence. Laboratories must prove they can execute specific test methods within defined scopes—not simply document that procedures exist.
1) Risk Reduction for Enterprise Clients
Measurement errors, inaccurate calibration, or unreliable testing create cascading business risks: product recalls, regulatory enforcement actions, customer contract breaches, and liability exposure. ISO/IEC 17025 accreditation mitigates these risks by requiring laboratories to maintain documented evidence of technical competence, implement systematic quality controls, and participate in proficiency testing that validates performance against peer laboratories.
2) Operational Efficiency Gains
Enterprises working with accredited laboratories experience smoother supply-chain qualification processes, accelerated quality control cycles, and reduced documentation burdens during customer audits or regulatory inspections. Auditors and procurement teams recognize ISO/IEC 17025 accreditation as sufficient evidence of laboratory competence, eliminating the need for redundant vendor assessments.
3) Global Market Access
These ILAC MRA signatory accreditation bodies carry identical acceptance across the globe. The MRA arrangement was designed with equal weight across all economies. The International Laboratory Accreditation Cooperation (ILAC) Mutual Recognition Arrangement ensures that accreditation from one signatory body is accepted internationally. For enterprises, this means a single set of test results satisfies regulatory requirements across multiple jurisdictions—reducing testing redundancy and compliance complexity.
Common Misconceptions & Clarifications
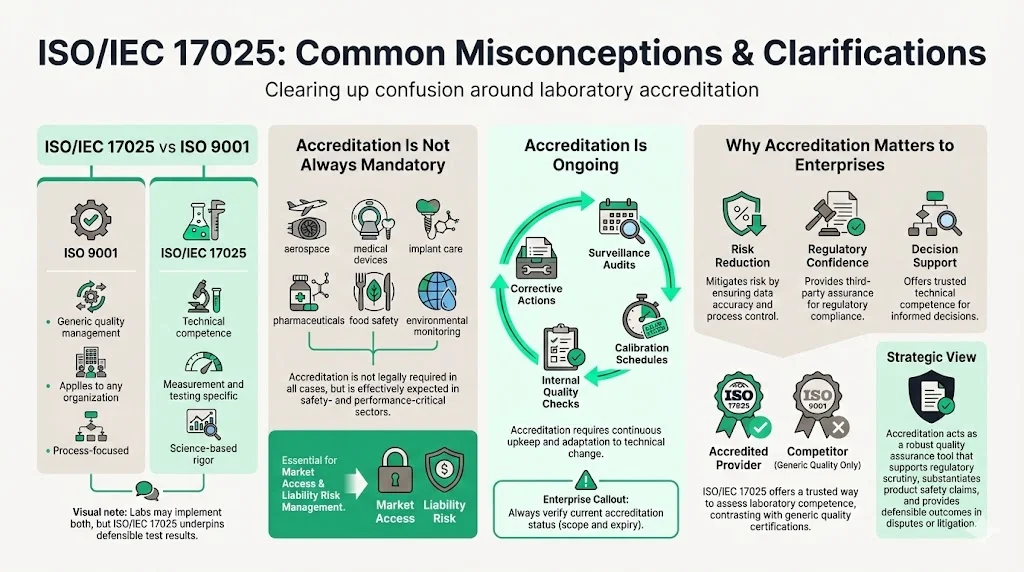
ISO/IEC 17025 vs. ISO 9001
There are many commonalities with the ISO 9000 standard, but ISO/IEC 17025 is more specific in requirements for competence and applies directly to those organizations that produce testing and calibration results and is based on more technical principles. ISO 9001 addresses generic quality management applicable to any organization; ISO/IEC 17025 focuses specifically on technical competence for measurement and testing activities. Laboratories may implement both standards, but 17025 provides the technical rigor necessary for defensible scientific results.
Accreditation Is Not Universally Mandatory
While many regulatory frameworks and enterprise procurement policies require ISO/IEC 17025 accreditation, it is not legally mandated in all contexts. However, industries where measurement accuracy directly affects safety, regulatory compliance, or product performance—aerospace, medical devices, pharmaceuticals, food safety, environmental monitoring—effectively treat accreditation as a baseline requirement. Organizations operating without accreditation face market access barriers and heightened liability exposure.
Accreditation Requires Continuous Maintenance
Achieving initial accreditation represents a starting point, not an endpoint. In common with other ISO quality standards, ISO/IEC 17025 requires continual improvement. Additionally, the laboratory will be expected to keep abreast of scientific and technological advances in relevant areas. Laboratories must undergo periodic surveillance audits, maintain equipment calibration schedules, document internal quality control results, and address corrective actions. Enterprises should verify that laboratories maintain current accreditation status rather than assuming historical accreditation guarantees ongoing competence.
ISO/IEC 17025 accreditation provides enterprises with a validated mechanism for assessing laboratory competence without requiring deep technical expertise in measurement science. For organizations supplying testing, calibration, or inspection services, obtaining accreditation differentiates technical capability from competitors who rely on generic quality certifications. For enterprises procuring these services, requiring ISO/IEC 17025 accreditation reduces risk, streamlines regulatory compliance, and ensures measurement accuracy underpins critical business decisions.
Decision-makers should treat ISO/IEC 17025 accreditation as a strategic quality assurance tool rather than a compliance formality. The standard addresses the fundamental question enterprises must answer when relying on external laboratories: Can we trust these results to withstand regulatory scrutiny, support product safety claims, and provide defensible evidence in contract disputes or litigation? Accreditation provides documented assurance that laboratories possess the technical competence, impartial processes, and systematic controls necessary to deliver reliable answers.
FAQs
1) What is the meaning of ISO 17025?
ISO/IEC 17025 is the international standard specifying general requirements for the competence, impartiality, and consistent operation of testing and calibration laboratories. It establishes criteria laboratories must meet to demonstrate they can produce valid, reliable results—enabling global acceptance of test reports and calibration certificates.
2) What is the ISO standard for calibration?
ISO/IEC 17025 serves as the primary international standard for calibration laboratories. The standard defines requirements for calibration processes, equipment traceability, measurement uncertainty estimation, and personnel competence. Calibration laboratories accredited to ISO/IEC 17025 demonstrate they can provide traceable measurements linked to national or international measurement standards.
3) What is the difference between ISO 17025 and ISO 13485?
ISO/IEC 17025 applies specifically to testing and calibration laboratories, addressing technical competence in measurement activities. ISO 13485 is a quality management system standard for medical device manufacturers and related organizations. While both standards support regulatory compliance in healthcare sectors, ISO 13485 governs product design, manufacturing, and distribution processes, whereas ISO/IEC 17025 focuses on laboratory testing and measurement competence. Medical device manufacturers often require suppliers to provide test results from ISO/IEC 17025-accredited laboratories while simultaneously maintaining their own ISO 13485 certification.
4) What are the general requirements for ISO 17025?
ISO/IEC 17025:2017 includes general requirements covering impartiality and confidentiality; structural requirements defining organizational accountability; resource requirements addressing personnel competence, equipment, facilities, and metrological traceability; process requirements for testing, calibration, and sampling methods; and management system requirements for document control, audits, and continual improvement. Laboratories must demonstrate both technical competence in specific measurement activities and systematic management of quality assurance processes.
5) What is equivalent to ISO 17025?
No direct equivalent to ISO/IEC 17025 exists with identical international recognition. Some sectors use specialized standards—ISO 15189 for medical laboratories, GLP (Good Laboratory Practice) for certain regulatory testing contexts—but these address different scopes or requirements. Regional or national accreditation schemes existed before ISO/IEC 17025 standardization, but most have converged on ISO/IEC 17025 as the baseline. The ILAC Mutual Recognition Arrangement ensures that accreditation from different national bodies carries equivalent weight internationally, but the underlying standard remains ISO/IEC 17025.
6) How much does ISO 17025 certification cost?
ISO/IEC 17025 accreditation costs vary significantly based on laboratory size, scope of accreditation (number of test methods or calibration parameters), geographic location, and chosen accreditation body. Direct accreditation fees typically range from several thousand to tens of thousands of dollars annually, covering initial assessment, surveillance audits, and administrative costs. However, total implementation costs—including quality system development, personnel training, equipment upgrades, documentation preparation, and consultant support—often exceed direct accreditation fees by factors of three to ten. Small laboratories pursuing narrow scopes may invest $15,000-$50,000 total; large multi-site laboratories with extensive scopes can exceed $200,000 for initial implementation. Ongoing annual costs for maintaining accreditation typically represent 20-40% of initial implementation investment.
7) How do you get ISO 17025 certified?
ISO/IEC 17025 accreditation—not certification—requires laboratories to follow a defined process. First, identify the appropriate national or international accreditation body recognized under the ILAC Mutual Recognition Arrangement. Second, define the scope of accreditation specifying which test methods, calibration parameters, or sampling activities require accreditation. Third, implement the standard's requirements: establish quality management documentation, validate test methods, calibrate equipment, train personnel, and conduct internal audits. Fourth, engage the accreditation body to conduct a formal assessment, including document review and on-site evaluation of technical competence. Fifth, address any nonconformities identified during assessment through corrective actions. Finally, maintain accreditation through periodic surveillance audits, ongoing proficiency testing participation, and continuous quality system maintenance. The process typically requires 12-24 months from initial planning to achieving accreditation, depending on laboratory maturity and scope complexity.



.svg)
.svg)
.svg)