Most organizations pursuing security certifications overlook a foundational reality: compliance frameworks like SOC 2 and ISO 27001 depend on underlying operational discipline. Without structured processes, evidence collection becomes chaotic, control implementation inconsistent, and audit readiness perpetually elusive. This is where ISO 9001 compliance provides strategic value—not as a replacement for security standards, but as the operational foundation that makes sustained compliance achievable.
Enterprise buyers increasingly evaluate vendors through comprehensive risk assessments that extend beyond security questionnaires. Procurement teams examine operational maturity, process consistency, and organizational reliability. ISO 9001 compliance signals disciplined execution—the ability to deliver predictable outcomes through controlled, repeatable processes. For technical leaders managing vendor relationships or preparing for enterprise sales cycles, understanding ISO 9001's role within broader compliance programs provides competitive advantage.
This definition clarifies what ISO 9001 compliance actually requires, how it differs from security-focused frameworks, and why quality management systems create leverage for organizations building comprehensive compliance postures.
What Is ISO 9001 Compliance?
ISO 9001 compliance means establishing, implementing, and maintaining a quality management system (QMS) that meets internationally specified requirements for delivering consistent products and services that satisfy customer and regulatory requirements. Unlike voluntary frameworks, ISO 9001 defines mandatory requirements organizations must fulfill to demonstrate quality management capability.
Within the ISO 9000 family of standards, ISO 9001 is the only standard that can be certified to, though certification itself remains optional. The ISO 9000 family includes foundational documents—ISO 9000 establishes terminology and principles, while ISO 9001 contains the actual certifiable requirements. ISO 9004 provides guidance on sustained organizational success, but only ISO 9001 serves as the basis for third-party certification audits.
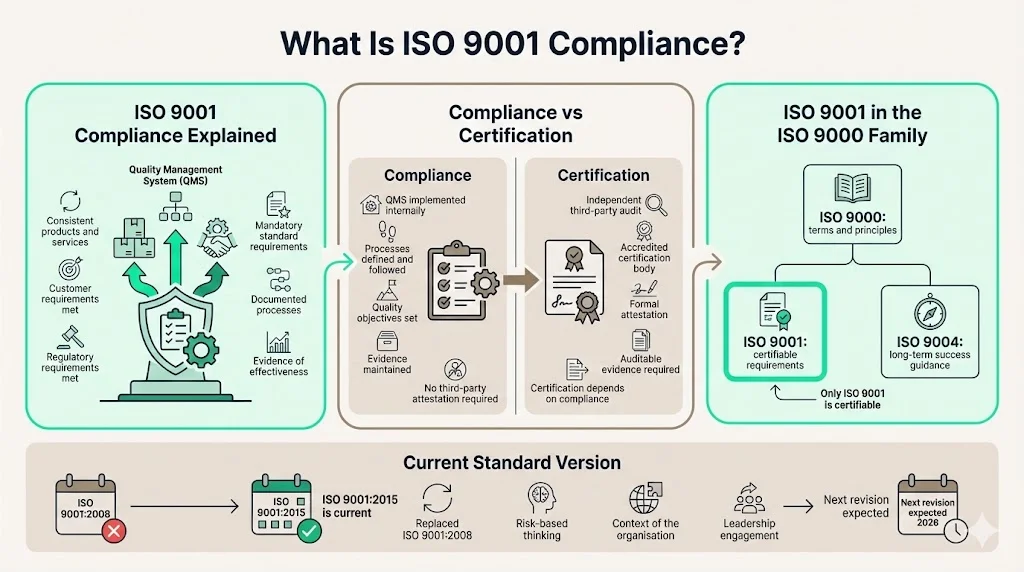
Compliance vs. Certification: Critical Distinction
Organizations frequently conflate compliance with certification. Compliance means implementing QMS requirements within operations—establishing documented processes, defining quality objectives, and maintaining evidence of control effectiveness. Certification represents formal attestation by an accredited third-party certification body that your implemented system meets ISO 9001 requirements. You can be compliant without certification, but certification requires demonstrable, auditable compliance.
ISO 9001:2015 represents the current version of the standard, having replaced ISO 9001:2008. The standard undergoes revision cycles and is expected to be published in 2026, with amendments addressing climate action considerations. Organizations implementing ISO 9001 today reference the 2015 version, which introduced risk-based thinking, context of the organization, and leadership engagement as explicit requirements.
Understanding ISO 9001 as a Quality Management System
What a Quality Management System (QMS) Really Means
A quality management system represents the documented framework of policies, processes, procedures, and resources an organization uses to direct and control quality-related activities. Rather than dictating specific operational methods, ISO 9001 requires organizations to define how they plan, execute, monitor, and improve processes that affect product or service quality.
The core premise: quality results from systematic process control, not inspection after the fact. Organizations document what they do, do what they document, and maintain evidence proving both. This discipline creates operational consistency—the foundation for predictable outcomes and audit readiness.
The Process Approach Explained
ISO 9001 mandates a process approach—viewing organizational activities as interconnected processes with defined inputs, outputs, and ownership. Each process transforms inputs (materials, information, requirements) into outputs (products, services, decisions) through controlled activities. Process owners bear accountability for performance, resource allocation, and improvement initiatives.
This approach differs fundamentally from functional organizational thinking. Rather than optimizing individual departments, the process approach examines how work flows across boundaries. A sales process spans marketing, engineering, operations, and finance—requiring coordination, handoffs, and shared objectives. ISO 9001 requires organizations to identify these processes, document their sequence and interaction, and establish criteria for effective operation and control.
The connection to business efficiency becomes apparent during implementation: process mapping exposes redundancies, bottlenecks, and unclear accountabilities. Organizations implementing ISO 9001 typically discover that 20-30% of operational activities provide minimal value or duplicate effort across teams—inefficiencies that persist because no one owns end-to-end process performance.
Core ISO 9001 Compliance Requirements### Leadership and Quality Policy
ISO 9001 places explicit accountability on top management—executives cannot delegate quality responsibility to operational teams. Leadership must establish and maintain a quality policy: a documented statement of quality objectives, commitment to meeting requirements, and dedication to continuous improvement. This policy requires communication throughout the organization and regular review for continued appropriateness.
Top management accountability extends beyond policy statements. Leaders must ensure customer focus drives decision-making, that quality objectives align with strategic direction, that adequate resources support QMS implementation, and that process owners possess authority to fulfill their responsibilities. During certification audits, auditors interview executives directly to verify leadership engagement—perfunctory involvement becomes immediately apparent.
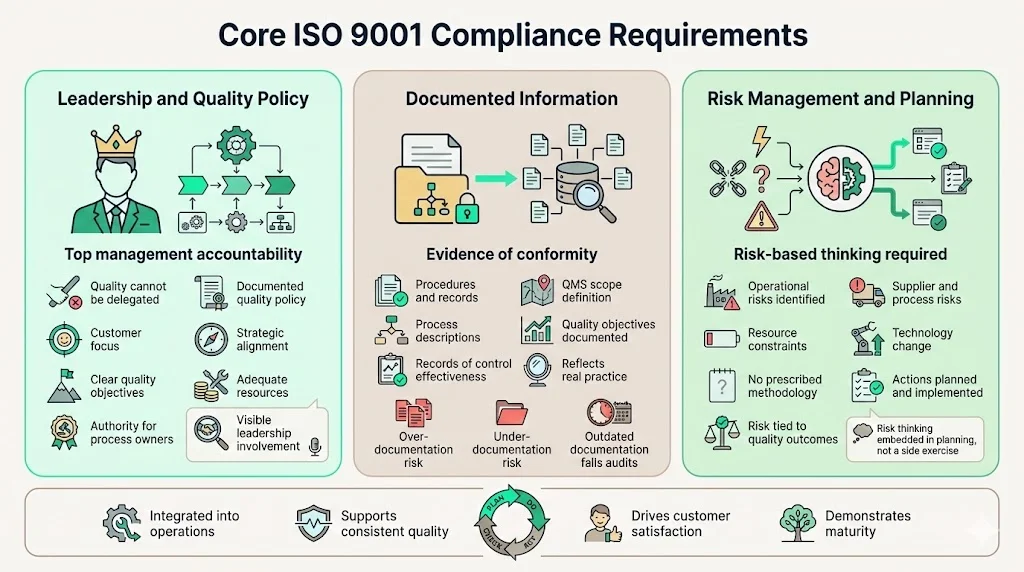
Documented Information
ISO 9001 uses the term "documented information" to encompass both documented procedures (how processes operate) and records (evidence that activities occurred). Organizations must maintain documented information demonstrating control over processes and conformity to requirements.
Required documentation includes quality policy, quality objectives, QMS scope definition, process descriptions sufficient to ensure effective operation, and records proving conformity and control effectiveness. Common documentation mistakes include over-documenting (creating procedures for every conceivable activity), under-documenting (relying on tribal knowledge without capturing critical processes), and maintaining documentation that doesn't reflect actual practice—a fatal audit finding.
The standard intentionally avoids prescribing documentation volume or format. A 50-person software company requires different documentation depth than a 5,000-person manufacturing operation. The key requirement: documented information must be sufficient to ensure consistent process execution and provide objective evidence during audits.
Risk Management and Planning
ISO 9001:2015 introduced explicit risk-based thinking as a core requirement. Organizations must identify risks and opportunities that could affect QMS conformity, product and service quality, and ability to enhance customer satisfaction. This extends beyond traditional business risk to encompass operational risks—supplier failures, process variations, resource constraints, technological changes.
Risk management requirements differ fundamentally from formal enterprise risk management programs. ISO 9001 doesn't mandate specific risk assessment methodologies, risk registers, or quantitative risk scoring. Instead, it requires organizations to think systematically about what could prevent achievement of intended outcomes, and to plan actions addressing those risks. A software company might identify technical debt accumulation as a quality risk requiring architectural review cycles. A manufacturing operation might address single-source supplier risk through qualification of alternate vendors.
The connection to organizational performance becomes evident during audits: organizations that integrate risk thinking into planning, resource allocation, and improvement initiatives demonstrate mature quality management. Those treating risk management as a separate compliance exercise inevitably maintain disconnected documentation that provides no operational value.
The ISO 9001 Certification Process
Step-by-Step Certification Overview
ISO 9001 certification typically requires 6-9 months from initial gap analysis through final certification, though timelines vary based on organizational size, existing documentation, and implementation discipline. The process begins with gap analysis—comparing current practices against ISO 9001 requirements to identify implementation needs.
Following gap analysis, organizations develop and implement required processes, create supporting documentation, train personnel, and begin collecting evidence of conformity. Internal preparation includes establishing quality objectives, defining process metrics, conducting internal audits, and executing management reviews. This implementation phase consumes the majority of the certification timeline—building genuine operational discipline cannot be rushed.
External certification involves two distinct audit stages. Stage 1 audits review documentation readiness, verify QMS scope appropriateness, and assess organizational understanding of requirements. Stage 2 audits examine actual implementation—auditors observe processes in operation, interview personnel, review records, and verify that documented procedures reflect real practice.
Audit Procedures Explained
Internal audits represent a mandatory ISO 9001 requirement—organizations must audit their own QMS at planned intervals to verify continued conformity and effectiveness. Internal auditors examine specific processes or departments, review documented information, interview process owners, and identify nonconformities requiring correction.
External certification audits follow similar methodologies but apply greater rigor. Auditors sample evidence across the QMS scope, trace specific transactions through complete process cycles, and verify that management review activities drive meaningful improvements. Auditors typically look for evidence demonstrating the Plan-Do-Check-Act cycle operates throughout the organization—planning processes, executing according to plans, monitoring performance, and taking corrective action when results fall short.
Nonconformities—instances where requirements aren't met—require corrective action but don't necessarily prevent certification. Minor nonconformities allow organizations to develop correction plans and demonstrate resolution before final certification issuance. Major nonconformities indicate systematic failures requiring immediate attention and re-audit after correction. Organizations treating audits as adversarial encounters rather than verification opportunities typically struggle—auditors assess whether your QMS operates as designed, not whether it matches some idealized theoretical model.
Continuous Improvement and Customer Satisfaction
How ISO 9001 Drives Continuous Improvement
ISO 9001 embeds continuous improvement as a core requirement through the Plan-Do-Check-Act (PDCA) cycle. Organizations must establish processes for identifying improvement opportunities, implementing changes, monitoring results, and standardizing effective improvements. This differs from aspirational improvement goals—ISO 9001 requires documented evidence that improvement activities occur systematically.
Using data and feedback to refine processes represents the "Check" phase of PDCA. Organizations collect quality metrics, monitor process performance indicators, analyze customer feedback, and review nonconformity trends. When data reveals performance gaps or improvement opportunities, corrective action processes kick in—requiring root cause analysis, implementation of preventive measures, and verification of effectiveness.
Customer Satisfaction as a Core Outcome
ISO 9001 positions customer satisfaction as a primary quality objective. Organizations must monitor customer perceptions regarding whether requirements have been met, using methods appropriate to their context—surveys, retention rates, warranty claims, complaint analysis, or direct feedback mechanisms.
Measuring customer expectations and outcomes requires clarity about what customers actually need versus what they initially request. A customer requesting expedited delivery may actually need predictable lead times allowing effective planning. ISO 9001 requires organizations to determine customer requirements, including implied needs and regulatory requirements applicable to products and services.
Complaint handling and corrective action processes provide enterprise clients visibility into organizational maturity. When issues arise—and they inevitably do—structured feedback loops ensure problems get escalated appropriately, root causes get addressed, and preventive actions preclude recurrence. Buyers evaluating vendors increasingly examine complaint resolution processes as indicators of operational discipline and customer-centricity.
ISO 9001 and Regulatory Adherence
ISO 9001 supports regulatory adherence by requiring organizations to determine and document applicable statutory and regulatory requirements relevant to products and services. This creates a systematic approach to compliance management—identifying requirements, translating them into operational controls, and maintaining evidence of conformity.
Aligning internal controls with industry regulations becomes more manageable within a structured QMS. Organizations subject to FDA regulations, automotive industry standards, or sector-specific compliance requirements can map those requirements to quality processes, ensuring regulatory obligations integrate into daily operations rather than existing as separate compliance programs.
This reduces compliance gaps during external reviews—whether customer audits, regulatory inspections, or certification assessments. When organizations maintain disciplined process control, documented procedures, and systematic evidence collection, demonstrating regulatory compliance becomes straightforward. The QMS serves as the operational backbone supporting multiple compliance objectives simultaneously.
Business Value of ISO 9001 for Enterprise Sales
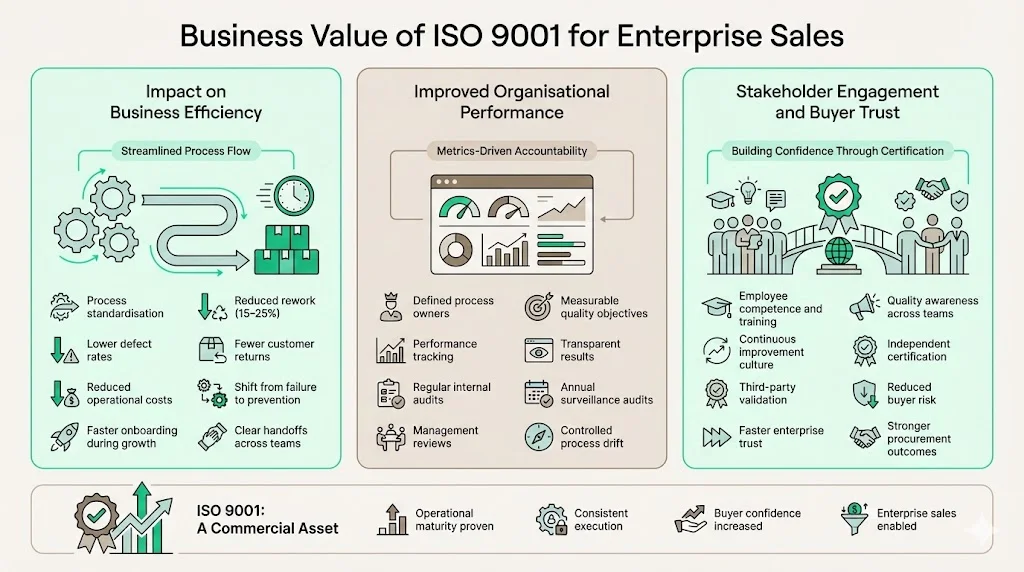
Impact on Business Efficiency
ISO 9001 implementation typically reduces rework by 15-25% through process standardization and error prevention mechanisms. When processes operate consistently, defect rates decline, customer returns decrease, and operational costs fall. Organizations measuring quality costs—prevention, appraisal, internal failure, and external failure costs—consistently observe favorable shifts toward prevention activities and away from failure costs.
Better cross-team coordination emerges naturally from process approach implementation. When marketing, engineering, operations, and finance understand their roles within end-to-end processes, handoffs improve, communication gaps close, and organizational silos diminish. This coordination becomes particularly valuable during scaling—new employees integrate more effectively when processes are documented, roles are clear, and quality expectations are explicit.
Improved Organizational Performance
Clear metrics and accountability represent fundamental ISO 9001 requirements. Process owners must establish quality objectives with measurable targets and monitor performance against those targets. This creates transparency around operational performance—making successes visible while exposing areas requiring improvement.
Stronger operational discipline follows from systematic process management. Organizations maintaining ISO 9001 certification can't allow processes to drift—surveillance audits occur annually, internal audits examine different areas quarterly, and management reviews assess overall QMS effectiveness at planned intervals. This creates continuous pressure toward disciplined execution and documented decision-making.
Stakeholder Engagement
ISO 9001 requires involving employees through competence requirements, training effectiveness verification, and awareness of quality policy and objectives. This engagement builds organizational quality culture—employees understand how their work contributes to quality outcomes and possess tools to identify improvement opportunities.
Building trust with enterprise buyers and auditors represents perhaps the most significant business value. When procurement teams conduct vendor assessments, ISO 9001 certification provides independent verification of operational maturity. Rather than relying solely on vendor claims, buyers can review certification scope, contact certification bodies, and request surveillance audit results. This third-party validation reduces buyer risk and accelerates trust development during enterprise sales cycles.
ISO 9001 Compared to Other Standards
ISO 9001 focuses exclusively on quality management—delivering consistent products and services that meet requirements. It addresses operational discipline, process control, and customer satisfaction, but contains no information security, privacy, or cybersecurity requirements. Organizations seeking comprehensive compliance programs require multiple frameworks addressing different risk domains.
ISO 9001 works alongside ISO 27001 (information security management) and SOC 2 (security and availability controls) through integrated management system approaches. Modern high-structure frameworks share common elements—context of the organization, leadership commitment, risk-based planning, operational controls, performance evaluation, and improvement. Organizations implementing multiple standards simultaneously can leverage this common structure, avoiding duplicative documentation while addressing distinct compliance domains.
Where quality management fits in enterprise risk programs depends on organizational priorities and customer requirements. Technology vendors selling to enterprises typically prioritize security certifications—SOC 2, ISO 27001, or FedRAMP—because buyers demand security assurances before considering product quality. Manufacturing organizations, medical device companies, or professional services firms often prioritize ISO 9001 because product quality directly impacts customer satisfaction and regulatory compliance.
The strategic insight: ISO 9001 provides operational foundation making security and compliance frameworks more achievable. Organizations lacking process discipline struggle to implement systematic security controls, collect compliance evidence, or maintain audit readiness. Quality management maturity creates leverage for comprehensive compliance programs.
Who Should Own ISO 9001 Compliance Internally
Leadership responsibilities extend throughout the executive team. The CEO or managing director bears ultimate accountability for QMS effectiveness. Functional leaders—VP of Operations, VP of Engineering, VP of Customer Success—own processes within their domains and must ensure adequate resources support quality objectives.
Quality managers or management representatives serve as QMS coordinators but don't own quality outcomes. Their role encompasses facilitating internal audits, maintaining QMS documentation, tracking corrective actions, and reporting on system performance to top management. Treating the quality manager as solely responsible for ISO 9001 compliance represents a fundamental misunderstanding—quality is everyone's responsibility, with the quality manager providing coordination and expertise.
Process owners bear day-to-day accountability for performance within their assigned processes. A process owner for order fulfillment ensures customer orders get processed accurately, inventory allocations occur correctly, and shipments meet promised delivery dates. Process owners establish process-level quality objectives, monitor performance metrics, and drive improvements when targets aren't met.
Frontline teams execute processes according to documented procedures, collect evidence of conformity, and identify improvement opportunities through their daily work. Their engagement determines whether the QMS represents genuine operational discipline or performative compliance. Organizations where frontline employees understand quality requirements and possess authority to escalate issues demonstrate mature quality cultures.
Coordination across departments becomes critical during implementation and ongoing operation. Cross-functional process improvement teams address issues spanning organizational boundaries. Management review meetings bring leadership together to assess overall QMS performance and allocate resources addressing strategic quality priorities.
Common Challenges in ISO 9001 Compliance

Over-documentation remains the most frequent implementation mistake. Organizations create detailed procedures for every conceivable activity, producing shelf-loads of documentation no one reads or follows. This stems from misunderstanding ISO 9001's actual requirements—the standard mandates documentation sufficient to ensure effective operation, not comprehensive documentation of every process detail. A procedure explaining "how to make coffee" signals over-documentation; a procedure defining "customer complaint escalation criteria" addresses genuine quality risk.
Treating compliance as a one-time task creates inevitable surveillance audit failures. Organizations rush to certification, implement minimum required processes, achieve certification, then allow systems to drift. ISO 9001 requires continuous operation—internal audits quarterly, management reviews at planned intervals, corrective actions when nonconformities occur. Certification represents proof of capability at a point in time; maintaining certification requires sustained discipline.
Lack of internal buy-in undermines even well-designed quality management systems. When leadership treats ISO 9001 as a certification requirement rather than operational improvement opportunity, employees view the QMS as bureaucratic overhead. This creates the worst possible outcome—organizations invest significant resources building systems that provide minimal operational value while generating compliance documentation to satisfy auditors.
Growing companies avoid these issues by treating ISO 9001 implementation as operational maturity development. Start with essential processes affecting product quality and customer satisfaction. Document what you actually do, not what you wish you did. Engage employees in process design—people executing work daily possess insights consultants and executives lack. Measure outcomes that matter to business performance, not just audit compliance. When quality management drives genuine operational improvement, sustained compliance follows naturally.
Conclusion
ISO 9001 compliance matters for companies selling to enterprises because it demonstrates operational maturity that security certifications alone cannot prove. Enterprise buyers evaluate vendors through comprehensive risk lenses—security capabilities, operational reliability, financial stability, and organizational discipline. ISO 9001 certification signals ability to deliver predictable outcomes through controlled, repeatable processes.
The long-term value extends beyond certification credentials. Organizations building genuine quality management systems develop operational capabilities supporting sustained growth—documented processes enabling efficient scaling, systematic improvement mechanisms addressing performance gaps, and cross-functional coordination reducing organizational friction. These capabilities compound over time, creating competitive advantages that pure-play compliance programs cannot replicate.
A strong QMS supports trust, scale, and repeatable delivery by establishing the operational foundation underlying all compliance initiatives. Security controls implemented within disciplined processes prove more reliable than controls layered atop operational chaos. Evidence collection becomes systematic rather than frantic pre-audit scrambles. Audit readiness transitions from periodic achievement to continuous state. Organizations approaching compliance through operational excellence rather than certification achievement build sustainable competitive advantage in enterprise markets.
Frequently Asked Questions About ISO 9001 Compliance
1. What does ISO 9001 compliance mean?
ISO 9001 compliance means implementing a quality management system that meets internationally specified requirements, demonstrating organizational ability to consistently provide products and services that meet customer and regulatory requirements. Compliance requires documented processes, defined quality objectives, systematic monitoring, and evidence of continuous improvement—not merely aspirational quality goals.
2. Is ISO 9001 mandatory?
ISO 9001 remains voluntary unless customers, contracts, or regulatory bodies make it mandatory. Many industries—aerospace, automotive, medical devices—require supplier certification as a condition of doing business. Enterprise procurement processes increasingly include ISO 9001 certification as vendor qualification criteria. While technically optional, market expectations often make certification practically necessary for companies pursuing enterprise sales.
3. How long does certification last?
ISO 9001 certification, the most popular ISO standard with more than 1 million certified users, operates on a three-year certification cycle. Organizations undergo initial certification audits, then face annual surveillance audits verifying continued conformity. After three years, recertification audits occur—essentially repeating the full certification assessment. Organizations maintaining effective quality management systems find surveillance audits straightforward; those allowing systems to drift face findings requiring corrective action.
4. Does ISO 9001 help with security controls?
ISO 9001 addresses quality management, not information security or cybersecurity controls. The standard contains no requirements for access control, encryption, vulnerability management, incident response, or security monitoring. Organizations requiring security certifications must pursue ISO 27001, SOC 2, or other security-focused frameworks. However, ISO 9001's process discipline creates a foundation supporting security control implementation—documented procedures, change management, and systematic monitoring apply across both quality and security domains.
5. Who should be involved in compliance?
Leadership teams bear ultimate accountability for quality management system effectiveness. Quality managers coordinate implementation and maintenance activities. Process owners manage day-to-day operations within assigned processes. Frontline employees execute procedures and collect conformity evidence. Effective ISO 9001 compliance requires engagement across all organizational levels—quality cannot be delegated to a single department or role. Organizations treating ISO 9001 as a quality department responsibility rather than enterprise-wide operational discipline inevitably struggle with sustained compliance and miss opportunities for genuine performance improvement.



.svg)
.svg)
.svg)